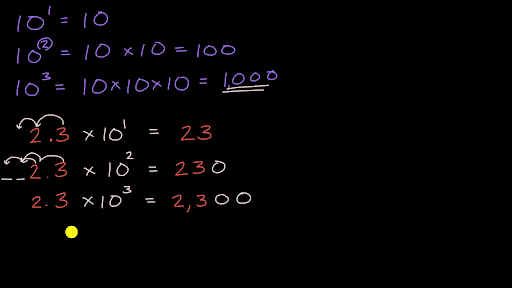Total kinetic energy of sample of a gas which contains 1 × 10^22 molecules is 24 × 10^2 J at 127^oC . Another sample of gas at 27^oC has a total kinetic
57.suppose a gas sample in all have 6*1023molecules. each 1/3 of the molecules have r.m.s. speed 104cm/sec, 2*104cm/sec, 3*104cm/sec. calculate the r.m.s. speed of gas molecules in sample

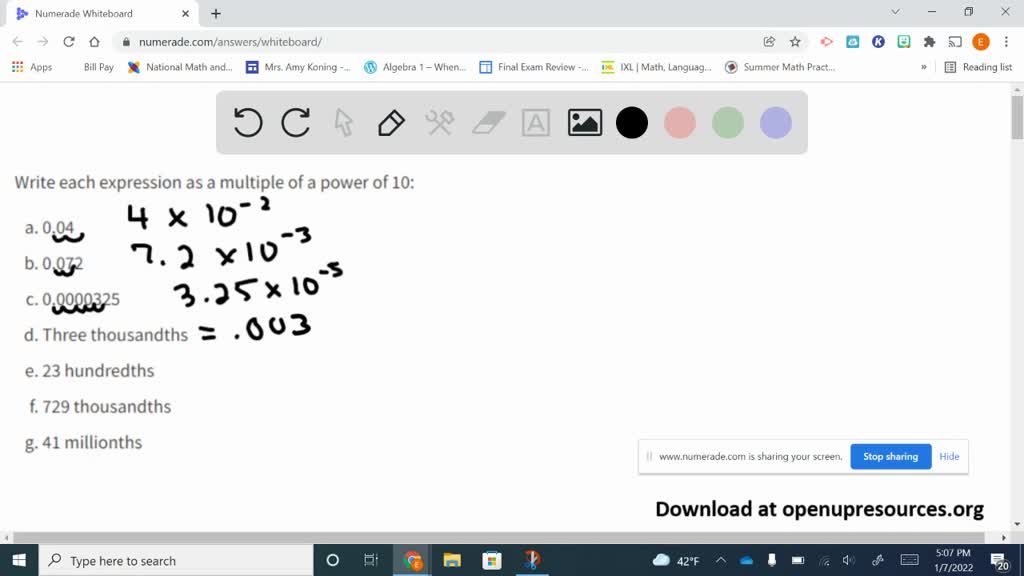
SF 03 - Multiplying by Powers of 10 | SF 03 - Multiplying by Powers of 10 The 4th in the Standard Form Series... Teaches how to multiply whole numbers and decimals

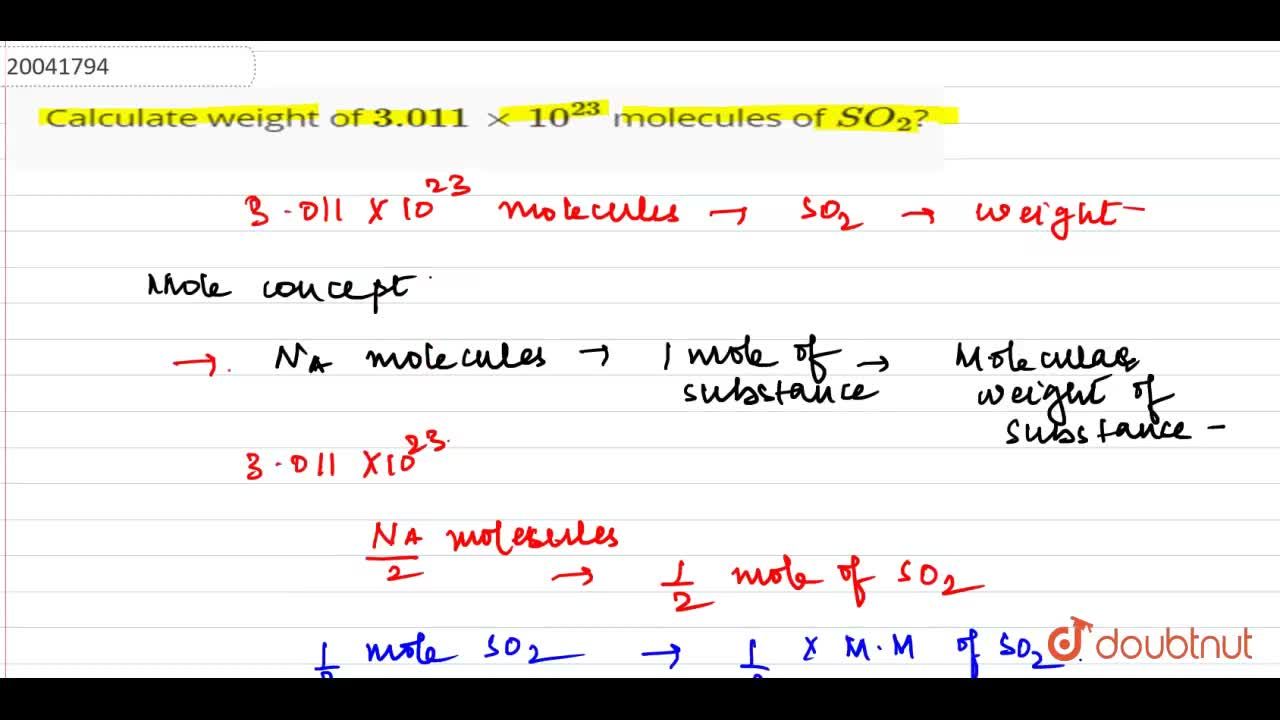
Calculate the number of moles for:a) 3.0115 × 10^23 atoms of copperb) 27.95 g of ironc) 1.51 × 10^23 molecules of CO2

Multiply 0.2 x 6.022 x 10^23 Need step by step explanation no direct answer or else get reported and ID - Brainly.in