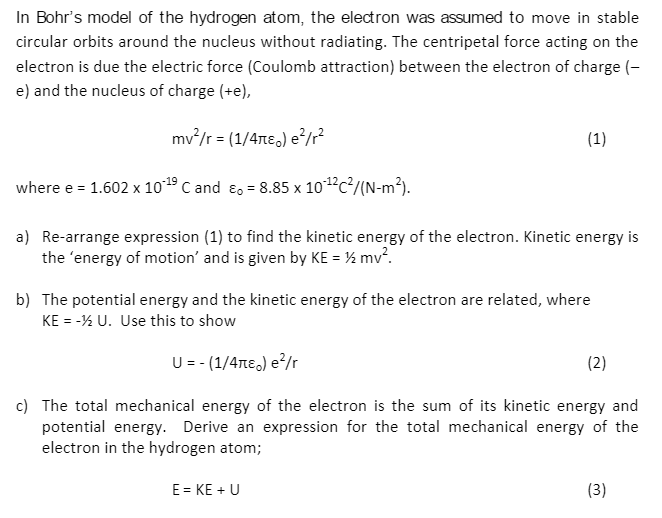
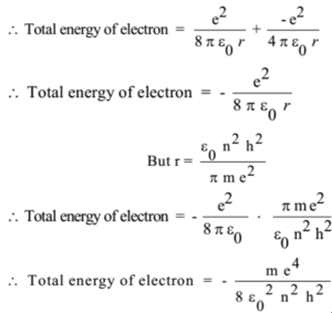
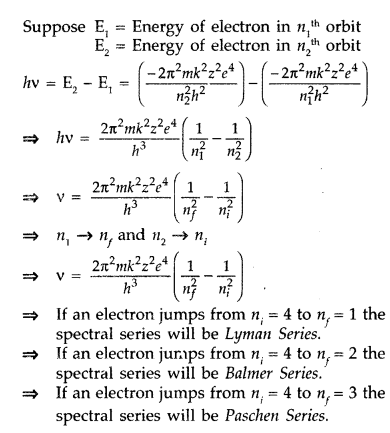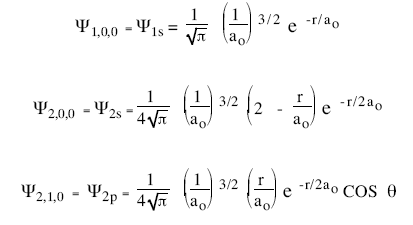Using Bohr's postulates, obtain the expression for (i) kinetic energy and (ii) potential energy of the electron in stationary state of hydrogen atom. - Sarthaks eConnect | Largest Online Education Community

The energy of an electron in the first Bohr orbit of H atom is `-13.6 eV` The potential energy v... - YouTube
What is the ratio of energy of the electron in the ground state of hydrogen to the electron in first excited state of He+? - Quora

using Bohr's postulates derive the expression for the frequency of radiation emitted when an electron - Brainly.in

The energy of the electron in the ground state of hydrogen atom is `-13.6 eV`. Find the kinetic - YouTube
i) Using Bohr's postulates, derive the expression for the total energy of the electron in the stationary states of the hydrogen atom. - Sarthaks eConnect | Largest Online Education Community
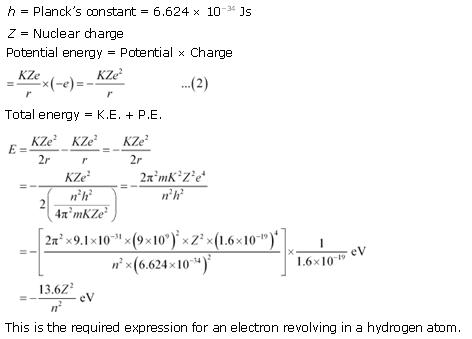
what are the three basic postulates of bohrs model of hydrogen atom derive an expression for the total energy of electron in bohrs stationary orbit - Physics - TopperLearning.com | t6nte4mxx
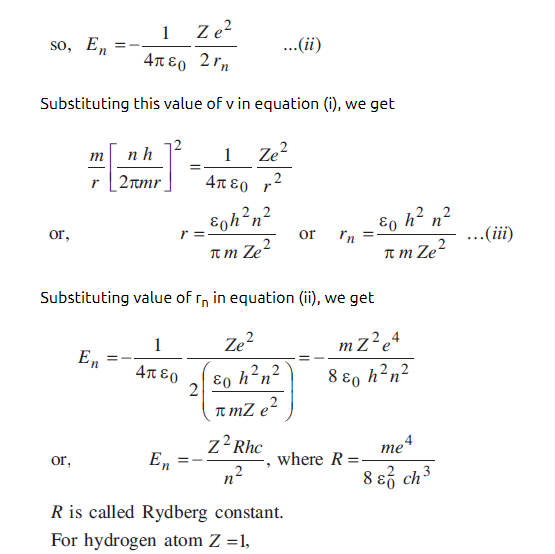
Using bohr postulate derive the expression for the frequency of radiation emitted by an electron in hydrogen atom undergoes transition from higher energy States to lower energy state? | EduRev Class 12 Question

For a hydrogen atom in its ground state, use the Bohr model to compute (a) the orbital speed - YouTube